Diabetic Foot Ulcer
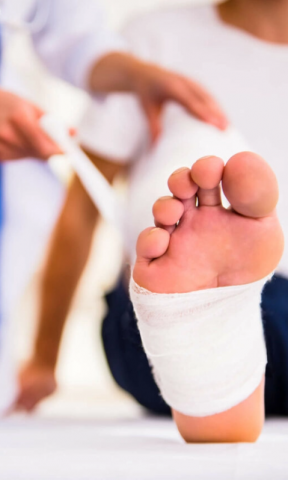
Disease Overview
Image

Image
GAP IN TREATMENT OPTIONS
Current Standard Of Care
Image
Debridement
Debridement should be carried out in all chronic wounds to remove surface debris and necrotic tissues.
Image
Off-loading
Off-loading of the ulcer area is extremely important for the healing of plantar ulcers.
Image
Dressings
Ulcers heal more quickly and are often less complicated by infection when in a moist environment.
Image
Bioengineered Skin Substitutes
Accumulating evidence shows that bioengineered skin substitutes may be a promising therapeutic adjunct therapy to standard wound care.
Image
MMP Modulators
Matrix metalloproteinases regulate the extracellular matrix components.
The management of DFU remains a major therapeutic challenge which implies an urgent need to review strategies and treatments.
DIABETIC FOOT ULCER
Mechanism of Action
Anti-inflammatory, angiogenic and wound healing properties of MSCs are key to successfully treat patients with DFU
Image
Clinical Trial Design
Phase 3 - A Label extension, Randomized, Double Blind, Placebo Controlled, Multicentre, Single Dose, Phase III Study Assessing the Efficacy and Safety of Peri-ulcer Administration of Stempeucel® (Adult Human Bone Marrow Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells) in Patients with Non-Healing Diabetic Foot Ulcer
- Proportion of patients with complete healing / closure of the target ulcer at any time during the 12 week treatment period with sustained complete closure for 12 additional weeks of follow-up (Time frame: 12+12= 24 weeks)
- Rate of reduction in size of the target ulcer
- Proportion of patients with at least 50% closure of target ulcer during the 24 weeks period
- Proportion of patients with complete healing / closure of the target ulcer at any time during the 24 week follow up period
Product Development Status
Diabetic Foot Ulcer
Indication
Basic R&D
Pre-Clinical
Phase 1
Phase 2
Phase 3
Marketing Authorization
Current status of Clinical Trials: All clinical trials are approved by DCGI in India